It is not unreasonable to have concerns about the cost of medicines.
Drug costs are usually influenced by government policies on pricing and reimbursement of medicines themselves. These range from simple discount seeking to more complex approaches such as conditional approvals, and value-based pricing (perhaps a subject for another posting). These can achieve a measure of drug cost control, but may also distort the market of medicines themselves.
For instance, tendering for generic medicines can sometimes lead to unacceptable consequences, such as unexpected product substitution by suppliers, patient and clinician confusion as medicines change appearance, and complications in medicines management or pharmacists. And a ‘winner take all’ award of contract can mean that the losers exit the market, removing a source of price competition and choice for consumers and governments. This is an unintended but avoidable consequence of using this crude procurement instrument.
Regulations and health technology assessment together are challenging to free pricing of medicines, but it is unsurprising that medicines should be subject to some assessment of efficacy and performance in the real world, and not just on the results of clinical trial evidence on a highly selected study population. HTA has also thrown into the spotlight the logic by which drug prices are established by the pharmaceutical industry. This scrutiny is not a bad thing as it highlights the methodologies used, whether they accurately produce a price reflecting the value of the medicine as used. Separately, the cost of the research to produce the medicine is a factor, and one should not be surprised that the prices of successful drugs should try to recoup the costs of all the failed drug research, even if those costs could be seen as the price of the risk of doing business for the industry.
Apart from these approaches to drug cost control, there are opportunities to reduce costs within the healthcare system itself.
Achieving improved cost control, value for money and improved health outcomes are consequence of better management of medicines procurement, patient adherence, dispensing and waste reduction and reduction in variations in prescribing practices.
These are processes and organisational interventions designed to enable improved professional practice through hospital formulary controls and best practice in medicines logistics. These enable the ability to reduce prescribing variance, strengthen quality systems and improve patient acceptability whilst strengthening the foundations of professional practice.
The following “logic map” shows how this works:
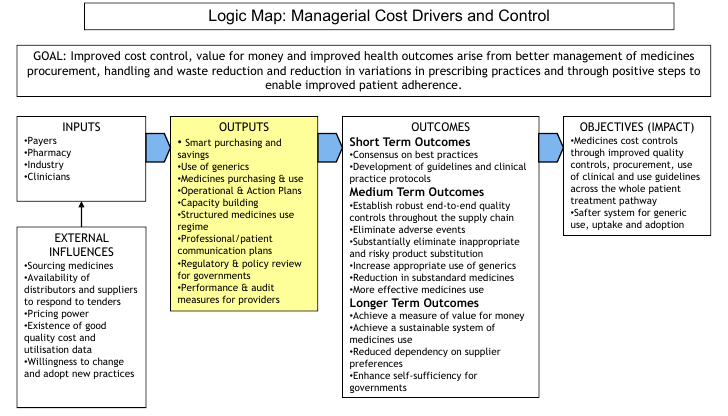
A central feature of any high-performing healthcare system or organisation includes best-practice in medicines use and clinical management.
As all aspects of healthcare are under varying degrees of financial stress, cost controls and appropriate use of medicines are a legitimate focus of scrutiny to achieve the highest standards of clinical practice and safe patient care.
Failure to achieve clinical and managerial control of the use of medicines across the patient treatment pathway may arise from:
- misuse of medicines (failure to prescribe when appropriate, prescribing when not appropriate, prescribing the wrong medicine, failure to reconcile medicines use across clinical hand-offs
- “clinical inertia” and failure to manage patients to goal (e.g. management of diabetes, and hypertension post aMI) [see for example: O’Connor PJ, Sperl-Hillen JM, Johnson PE, Rush WA, Blitz WAR, Clinical inertia and outpatient medical errors, in Henriksen K, Battles JB, Marks ES et al, editors, Advances in Patient Safety: From Research to Implementation Vol 2: Concepts and Methodology), Agency for Healthcare Research and Quality, 2005]
- failure to use or follow best-practice and rational prescribing guidance
- lack of synchronisation between the use of medicines (demand) and procurement (supply), with an impact on inventory management and
- loss of cost control of the medicines budget.
The essential challenge is ensuring that the healthcare system and its constituent parts are fit for purpose to address and avoid these failures or at least minimise their negative impact.
Medicines costs are the fastest growing area of expenditure and comprise a major constituent of patient treatment and recovery.
The cost of drug mortality was described in 1995 [Johnson JA, Bootman JL. Drug-related morbidity and mortality; a cost of illness model. Arch Int Med. 1995;155:1949/56] showing the cost of drug mortality and morbidity in the USA and costed the impact at $76.6 billion per year (greater than the cost of diabetes).
The study was repeated five years later [Ernst FR, Grizzle A, Drug-related morbidity and mortality: updating the cost of illness model, J Am Pharm Assoc. 2001;41(2)] and the costs had doubled.
And costs and use have continued to rise since then.
Evidence from a variety of jurisdictions suggests that drugs within the total cost of illness can be substantial, for instance:
- Atrial fibrillation: drugs accounted for 20% of expenditure [Wolowacz SE, Samuel M, Brennan VK, Jasso-Mosqueda J-G, Van Gelder IC, The cost of illness of atrial fibrillation: a systematic review of the recent literature, EP Eurospace (2011)13 (10):1375-1385]
- Pulmonary arterial hypertension: drugs accounted for 15% in a US study [Kirson NY, et al, Pulmonary arterial hypertension (PAH): direct costs of illness in the US privately insured population, Chest, 2010; 138.]
There are upward pressures that increase costs, downward pressures that decrease costs and pressures that influence costs in either direction; the diagram illustrates a few:
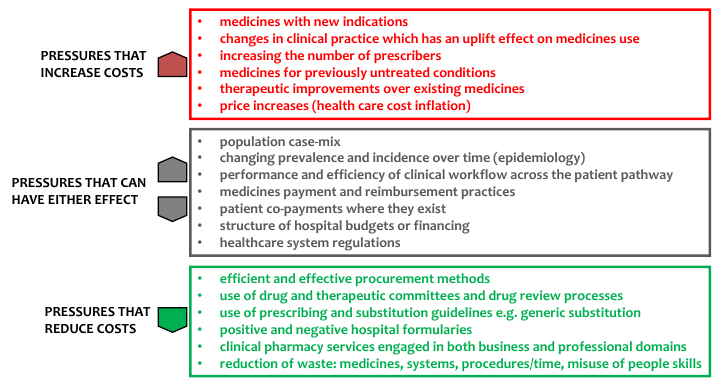
Many of the drivers can be addressed through a combination of professional staff development, better use of information, particularly within decision-support systems to support guidelines and prescribing compliance, and organisational interventions.
An interventional strategy to manage medicines cost drivers involves a structured review of central drivers of drug cost and use within existing national or organisational priorities.
The range of possible solutions fall across of spectrum of interventions and any or all of these are good starting points:
- development of drug use policies
- development of clinical policies, guidelines, and clinical decision-support algorithms
- drug-use evaluation studies
- clinical and medical audit
- cost-benefit studies
- professional development
- procurement effectiveness performance review
- patient treatment pathway analysis
- analysis of waste reduction opportunities
- management/organisational improvements to support appropriate behaviours.
To start involves assessing the current state of these aspects, and determine any gaps with national or organisational policy, or evidence-informed best practice. As a proxy measure of the necessary changes, measurement of this gap becomes the focus, and requires evidence of current practice against the desired goal. In many cases, where systems are weak or poorly performing a comprehensive root-and-branch review may be needed, with a corresponding impact on existing managerial, organisational and professional practice.
All healthcare systems and organisations are different and whilst it is difficult to precisely quantify the outcomes in advance, organisations undertaking a sustained process of medicines review and optimisation should be able to release more than 10% of existing drug expenditure and possibly more.
In organisations with a less-well developed clinical pharmacy, where medicines information systems are not well developed, and where clinical guidance is not proceduralised, greater savings are likely, perhaps to 25% or more, reflecting the possibility that the lack of information conceals upward drivers of costs, masks inefficient medicines management or evidence of misuse and waste.
In the longer run, healthcare organisations will need to ensure sustainability of any medicines optimisation review, by ensuring strong organisational structures, practices and behaviours. Development of these frameworks is an important by-product of medicines optimisation interventions, with a corresponding improvement in medicines safety.